HSCT: what to expect
HSCT is a hugely promising treatment for MS, but it is also very intense. This means that it comes with risks and there are lots of factors to consider.
If you're considering having HSCT, we recommend you talk to your neurologist about whether it's the right treatment for you.
Read our tips for talking about HSCT with your doctor or nurse
- Do I qualify to get HSCT on the NHS?
- What happens during HSCT?
- What should happen after HSCT treatment?
- How do I get HSCT?
- COVID-19 and HSCT
- Getting HSCT and the MS Society
Do I qualify to get HSCT on the NHS?
The NHS have criteria for who can get HSCT. These criteria refer to guidance from the European Society for Blood and Marrow Transplantation (EBMT).
But they don’t match up completely and there might be differences in how the guidelines are applied at the centres offering HSCT in Sheffield and London.
Clinical trials for HSCT might have slightly different criteria.
EBMT guidance for relapsing MS
HSCT should be offered to people with relapsing MS who:
- have had at least 2 relapses (or 1 relapse with signs of new lesions on MRI) in the previous 12 months
- have not responded to 1 or more existing DMTs(note - the NHS criteria says 2 DMTs)
- have an EDSS of 5.5 or less
- are younger than 45
- have had MS for less than 10 years
EBMT guidance for progressive MS
HSCT should be considered for people with primary and secondary progressive MS who:
- have evidence of their disability getting worse in the previous 12 months
- show signs of active inflammation (relapses or signs of new lesions on an MRI)
This should preferably be in a clinical trial.
People with ‘aggressive’ MS can also be considered. ‘Aggressive’ means you’ve developed severe disability in the previous 12 months. For aggressive MS, HSCT can be considered before trying another DMT.
NHS criteria for HSCT
The NHS criteria for HSCT in England refer to the EBMT guidance.
But the NHS says HSCT should only be used if 2 other DMTs haven’t worked (the EBMT says 1 DMT).
If you live in Wales, Scotland or Northern Ireland, you might be able to have the treatment at English hospitals that offer it.
Read the full EBMT guidelines for HSCT on nature website
Read the full NHS criteria for DMTs, including HSCT (PDF 531KB)
What happens during HSCT?
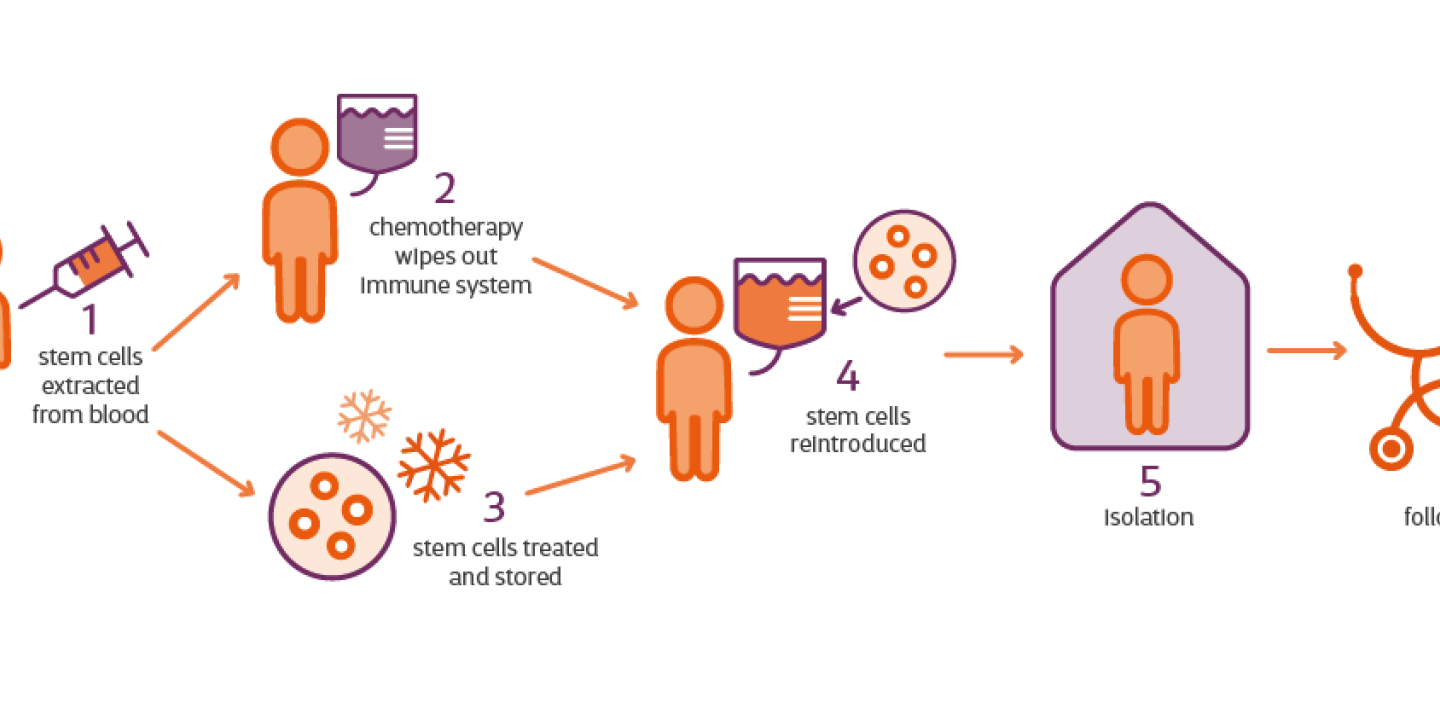
There are several steps to HSCT treatment for MS:
A specialist team reviews your case
Anyone being considered for HSCT will first be reviewed by the specialist team at the treatment centre.
Drugs get your stem cells ready for collection
The treatment begins with drugs to encourage stem cells to move out of your bone marrow and into your blood. This is known as mobilisation, and allows them to be collected. The drugs include chemotherapy and ‘G-CSF’ injections. G-CSF injections are manmade versions of a protein found naturally in our bodies. The symptoms of MS can get temporarily worse during this stage.
Your stem cells are collected
Around 10 days later, when there are enough stem cells in your blood, they will be removed and stored for later in the procedure.
Chemotherapy wipes out your immune system
Next, you'll be given a chemotherapy infusion to wipe out the immune system. This part of the procedure can take several days.
Chemotherapy may be myeloablative (completely wipes out the immune system) or non-myeloablative (partially wipes out the immune system). The side effects of chemotherapy include nausea and vomiting, so you might be given drugs to control this.
Your stem cells are returned to your blood
Finally, your stem cells are transplanted back into your blood by a drip to help regrow the immune system. This usually takes place a couple of days after the chemotherapy, once the drugs have cleared your system.
The stem cells get to work
The stem cells start making new blood and immune cells within 10 and 30 days of the transplant. As your immune system isn't working yet, you are more likely to get infections during this period. You're usually put on antibiotics and transfusions to support you.
Recovery from HSCT
Anyone having HSCT also has to spend around a month in an isolation room while their immune system rebuilds. This isolation can be lonely and challenging.
Read how three people who've had HSCT dealt with the isolation
HSCT is an intense treatment, so recovery can take some time. Typically, people need between 3 and 6 months to recover from HSCT. But for some people, it can take more than a year to fully recover.
What should happen after HSCT treatment?
- The transplant unit should organise regular outpatient monitoring in the first 2 years following the procedure and afterwards if needed
- Patients should be able to self-refer into a haematology department or another department that's familiar with HSCT, any time, 7 days a week, 24 hours a day
- The risks of infection persist for long periods after HSCT and antibiotics are recommended for many months, or sometimes even life-long
- Long-term monitoring should be backed up by patient data being held by the European Bone Marrow Transplant Registry (EBMT)
How do I get HSCT?
There are different ways of getting HSCT for people in the UK, including through the NHS and as part of a clinical trial. We’re working to help people with MS understand these routes and to get access to the right treatment for them.
On a clinical trial
You might be able to get HSCT on a clinical trial. A new trial called StarMS is now recruiting people with 'active' relapsing remitting MS. It's comparing HSCT with the highly effective DMTs alemtuzumab, ocrelizumab, ofatumumab and cladribine.
Find out more about the StarMS trial on the researchers’ website
Through the NHS
HSCT is available on the NHS. At the moment, it’s only considered for people who meet very specific medical criteria.
The main hospitals offering HSCT for MS in the UK are Sheffield and London (King’s College, Imperial College, University College London Hospitals and Barts Health Trust).
The criteria might vary slightly between them, so it’s worth checking with your neurologist about which place would suit you best.
People in Wales, Scotland and Northern Ireland who qualify to get HSCT might be able to have the treatment at these English hospitals.
If you don’t meet the criteria used by these hospitals, you could speak to your neurologist about getting HSCT through an individual funding request.
Going private in the UK
You can have HSCT privately at the centres in Sheffield and London. Again, these might have different rules about who qualifies for HSCT.
Going abroad
If you don’t qualify for HSCT on the NHS, it’s possible to get the treatment abroad. This option is very expensive. HSCT is available privately in several countries including Mexico, Russia, Israel and India.
It’s important to check the safety record of any centre. The EBMT have safety standards which they recommend for HSCT centres.
We'd urge anyone thinking of going abroad for treatment to first talk their options through with their MS nurse or neurologist.
Read more about having HSCT outside the NHS
COVID-19 and HSCT
During the pandemic, the Association of British Neurologists (ABN) advised against having HSCT except in exceptional circumstances because of the increased risk of infection.
But more recent EBMT guidance in May 2021 says HSCT can be carried out safely if people have a risk assessment. This risk assessment needs to take into account the local level of COVID-19.
This EBMT guidance also says people with highly active relapsing MS can have HSCT even when COVID-19 rates are high or rising.
Read the EBMT guidance for HSCT in the time of COVID-19
Getting HSCT and the MS Society
We’re working alongside clinicians to help make sure people with MS can get treatments that are right for them at the right time. This includes HSCT for people who are eligible.
We advocated for HSCT to be available on the NHS. We were part of an NHS England working group that developed treatment guidance which made HSCT available for the NHS in England for the first time. It's unusual for an unlicensed treatment to be made available and we were pleased to play a part in making this happen.
We played a key role in getting the people together who developed the new phase III clinical trial, Star-MS, which started recruiting participants in 2022.
The Scottish Health Technology Group is now looking at what’s needed to develop centres that can carry out HSCT in Scotland, and we hope to have a decision on this soon. In Wales and Northern Ireland we’re aware of a small number of people being referred for HSCT in England, but access remains very limited.
Last full review:
We also update when we know about important changes.