Behind the headlines: could an anti-histamine help repair myelin in MS?
Today, there have been reports in the news that a common anti-histamine might be able to repair myelin in MS. We look at the evidence.
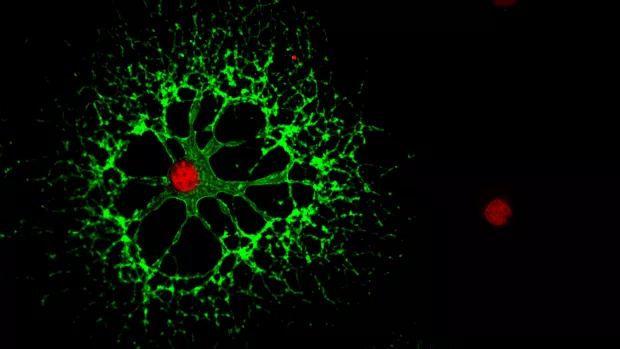
Myelin is the protective coating around our nerves. And finding ways to repair myelin is a key goal in our search for treatments to stop MS progression.
Clemastine is an active ingredient in anti-histamines. The idea of it having potential as a myelin repair treatment for MS is not new. But we don't yet know what its effect on MS is.
What did we already know about clemastine in MS?
In 2017, a small phase 2 clinical trial showed clemastine improved the speed at which messages travelled from the eye to the brain.
The ReBUILD trial involved 50 people with relapsing MS. One group took clemastine alongside a disease modifying therapy (DMT) for three months. The other group took only the DMT at first, and then added clemastine for two months.
The researchers suggested the improvement in the speed of messages indicated clemastine was improving myelin repair. But they didn’t see an effect on myelin repair when they looked at MRI scans of the whole brain.
Read the full results of ReBUILD on the journal website
What does the latest study show?
The latest study being reported in the news looked back at the results from the original ReBUILD trial. They wanted to know if there was evidence clemastine was having an effect on myelin repair, using a special type of MRI analysis.
This time, they looked at the amount of myelin in specific parts of the brain, known to have a lot of myelin.
They found the amount of myelin did increase in an area called the corpus callosum when people were taking clemastine. And it continued to increase for some time after people stopped taking it.
Read the full results of the new MRI study on the journal website
The results of the bexarotene trial already provided evidence from MRIs that a drug could boost myelin repair in people with MS. But this new study uses a different MRI technique to provide evidence of myelin repair taking place.
What’s next for clemastine research?
In 2022, a new MS Society-funded trial began exploring clemastine. This trial is based at Addenbrookes Hospital in Cambridge and is led by Dr Nick Cunniffe and Professor Alasdair Coles.
They’re testing clemastine in combination with another drug, metformin. Evidence from animal studies suggests metformin might enhance the effects of clemastine on myelin repair. So the researchers want to know if the combination could help repair myelin damage in relapsing MS.
The team need to recruit 70 people to take part in the trial. And they’re about half way through recruitment. All clinical trials need to have eligibility criteria, to give them the best chance of seeing an effect of a drug. In this trial, people taking part need to:
- Have relapsing remitting MS
- Be aged between 25 and 50 years
- Be stable on an MS DMT
- Live within three hours of Cambridge
Everyone takes part in the trial for six months, so we expect to see results before 2025.
Find out more about the trial through the Be Part of Research website
Why do we need these trials?
Testing existing drugs is an important way of speeding up how long it takes to find new treatments for MS. They may already be used safely in other conditions, but we don't yet know their effects on MS.
We need trials to tell us whether a drug has an impact on MS and if it has side effects. Without large clinical trials showing a drug is safe and effective, treatments can’t be licensed and made available on the NHS.
A carefully controlled clinical trial is the safest way for people to take unproven drugs. The drugs themselves are designed to be exactly the right formula and dose. And your health is closely monitored by a team of neurologists and MS nurses.